Water
Simple, Polar Molecule - Vast, Complex Chains
Home!

Bending Water, Electro-statically
Now, I may be a bit of a middle-aged academic, but I can still do the odd experiment every now and again. So what I'm doing is I'm charging up this
Perspex rod. So giving it an electric charge by rubbing it on the fleece. Now, watch what happens… When I put the rod next to a stream of water. Look
at that. The electric field, the electric charge, is bending the water towards it.
Now, the reason for that, the reason that water behaves in that way when it's passing through an electric field, is exactly the same reason that it
is vital for all life on Earth.
Water is a polar molecule, which means it responds to electric charge. Its polarity comes about because of the structure of water molecules themselves.
Now, water is H2O, two hydrogen is and one oxygen atom bound together.
So two hydrogen atoms approach oxygen. Now, oxygen's got a cloud of eight electrons
around it, so when the hydrogens come in, then what happens is the electrons get dragged over here, around the oxygen.
So you end up with an electron
cloud around here and, to some extent, pretty isolated, positively-charged protons out here.

Babbling Brook
So you get a net positive charge over here and the electron
cloud with its negative charge over here, so you get what's called a polar molecule. And that's why, when you bring a charged Perspex rod close to
water molecules, they bend towards it.
Water's polar nature means that although its molecules are simple, together, they form a subtle, endlessly complex liquid. A home in which one tiny
creatures thrives.

Pond Skater
There he is. Look at that. That… Is a pond skater. A predator that floats on the surface of the water and actually uses the surface of the water
to sense its prey. Pond skaters are vicious predators that live for most of their lives on the surface. Tiny hairs on their legs provide a large
area that spreads their weight. Their middle legs thrust them forward. Hind legs are employed to steer. They are so well adapted to life in this
flat world that they even sense their sexual partners through tiny vibrations on the water's surface.
The reason it can do that is the result of a complex interaction between adaptions in the animal itself and the physics and the chemistry of the
surface of water.
Water molecules are polar. And that means that water molecules themselves can bond together. So you can get a hydrogen with its slightly positive
charge getting close to the oxygen of another water molecule with its slightly negative charge and bonding to it. You can build up quite large, in
fact, VERY large structures in liquid water. This is what gives water its unique ability to form a surface habitat for the pond skaters.

Hydrogen Bonds
Clumps of H2O stick together, keeping the surface under tension. Forming a chorus of water molecules, all joined together by hydrogen bonds.
Then a pond skater comes along and puts its legs or its… Dangly things into the water and pushes it down, bends the surface of the water.
Now, the water doesn't like that because a bend in the water is increasing its surface area. Its increasing its energy. It's making it harder for
all the molecules to bond together with the hydrogen bonds. So they try to push back. They exert a force on the pond skater's leg because they want
to bond as much as they can. And that's how pond skaters stay on the surface of the water.
Hydrogen bonds do far more than just give the pond skaters a place to live. They are fundamental to all life. I've heard it said that we won't
truly understand biology until we understand water.

Capillary Action
These are… very thin tubes of glass. They're about a millimetre in diameter. And if I dip one into the surface of this river… can you see that
the water just climbs up the tube? It pulls itself up, quite literally, against the force of gravity. Now, in trees, there are tubes which are
about half the diameter of this, perhaps about half a millimetre or even less. And they are called xylem. And they allow the tree to lift water
up through the root system because the water molecules strongly attract each other and are strongly attracted to the sides of the tubes. So
when you look at trees like that, which are very high, and you ask yourself the question, "How do they get the water from the roots to the
top of the tree?", a big part of that is capillary action, which is down to the polar nature of water.

High Trees
One of water's most important qualities is its ability to dissolve and carry all manner of substances around the living world. Because its molecules
are very small and polar, water is a tremendously effective solvent. Those of molecules can get in amongst other substances, salts and sugars, for
example, and disperse them, if you like, in that sea of hydrogen bonds.
Within every one of us, water is constantly flowing around each and every cell. Blood plasma is over 90% water. And in it are dissolved everything I
need to live – oxygen, the nutrients from food, everything – distributed around my body in rivers of water.
We all live on a beautiful blue anomaly of a world. The only planet we know with a surface drenched in liquid water.
The story of how each drop ended up here has been hard to fathom. Largely because it happened so long ago, there is very little direct evidence.
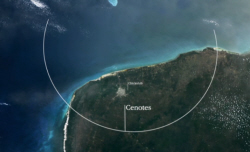
But back in the Yucatán jungle, clues to how it turned up can still be found. Every civilisation on the Yucatán, be it the modern Mexicans or the
Mayans, had to get their water from these deep wells, the cenotes. And I've got a completely unbiased map of the larger cenotes here, which I'm going
to overlay on the Yucatán.

Perfect Arc
Look at that. They lie in a perfect arc, centred around a very particular village, which is… there, and it's called Chicxulub. Now, to a
geologist, there are very few natural events that can create a structure, with such a perfect arc as that.
Footprint of Asteroid Strike
All the evidence points to just one explanation. You're looking at what's left of a gigantic asteroid strike. One that wiped out three-quarters of
all plant and animal species when it hit the Earth 65 million years ago.
You may think that impacts from space are a thing of the past. A thing that only happened to the dinosaurs, but that's not true. About 55 million kg
of rock hits the Earth every year. And around 2% of that is water. This hints that at least some of Earth's water arrived from space.

Hartley II
Late in 2010, these glimpses of comet Hartley 2 arrived back on Earth. They were sent by NASA's deep-impact probe. From its surface, dust and ice
spray into space. Analysis of this water found it had a very similar mixture of isotopes to the water in our own oceans.
This was the first firm evidence that icy comets must have contributed to the formation of our world's oceans.








